Through carbon offsetting Luforbec 100/6 and 200/6 pMDI are certified as Carbon Neutral products by Carbon Footprint Ltd.2,3

To read more about how carbon neutrality for Luforbec is achieved, click here.
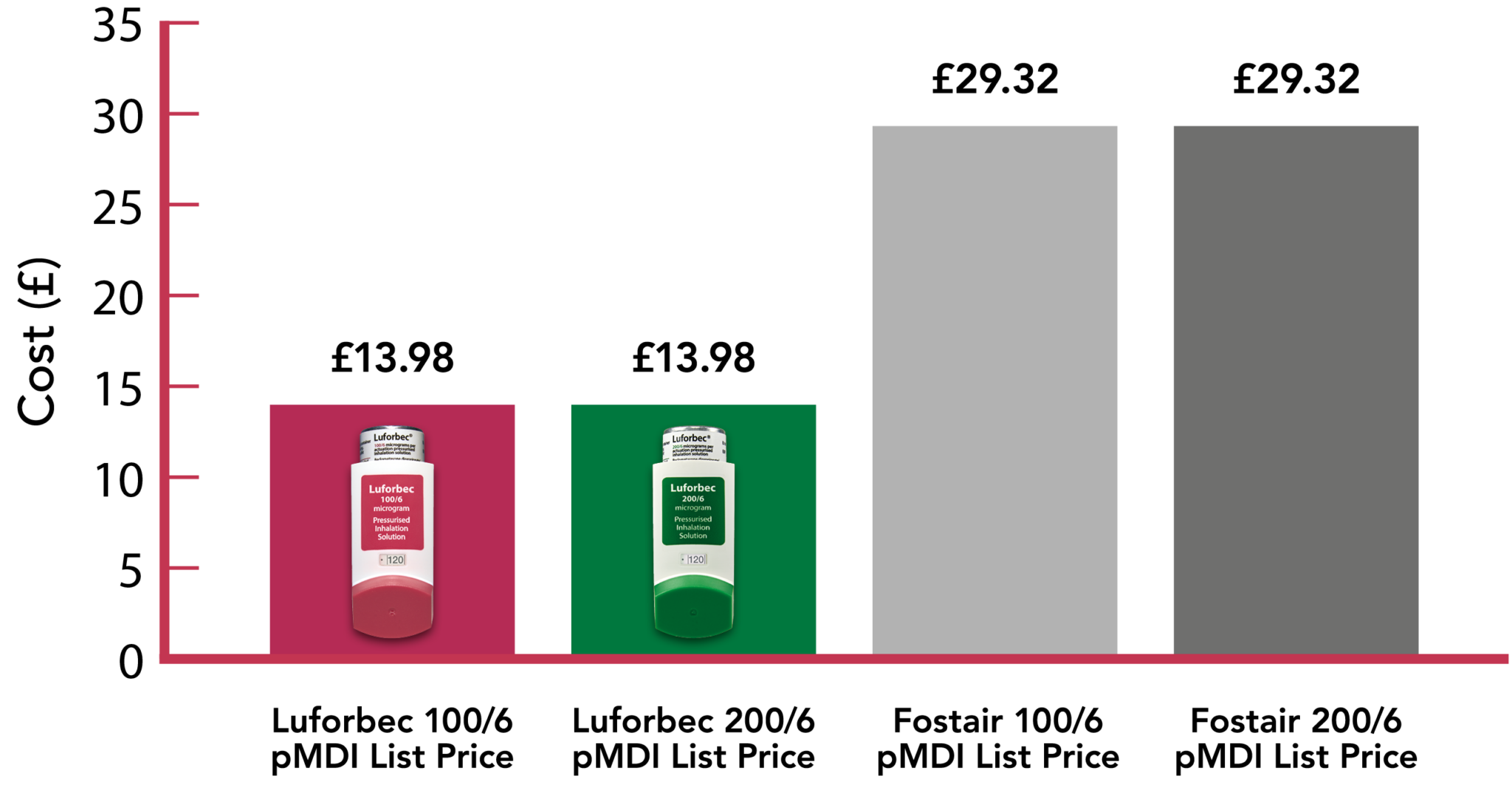
Luforbec offers an NHS List Price saving of 52% vs Fostair 100/6 and 200/6 pMDI1
 Prescribing Luforbec by brand could deliver cost
Prescribing Luforbec by brand could deliver costimprovements of up to £122.6 million per year 4
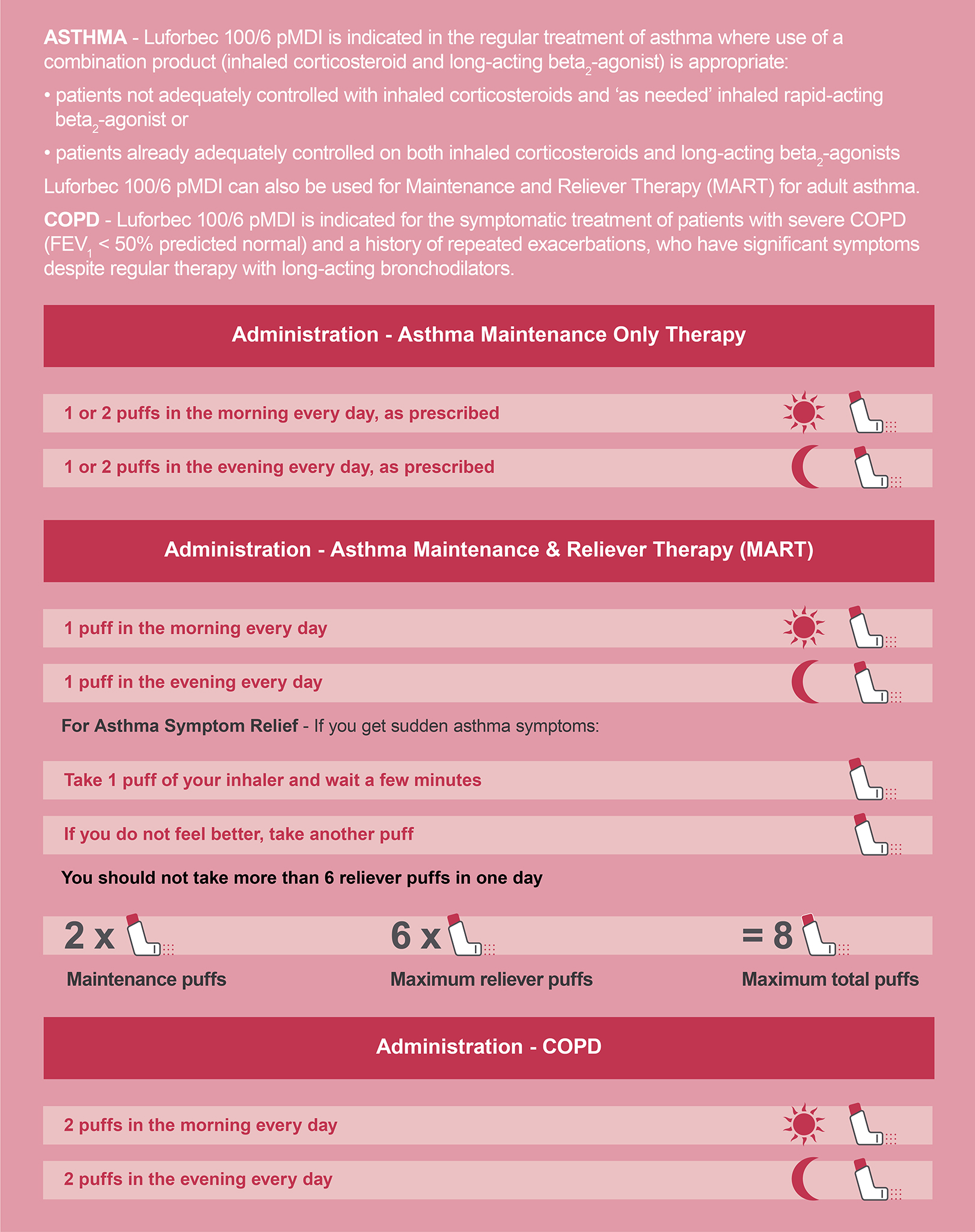
Beclometasone/Formoterol pMDIs are listed as Category C medicines in the NHS Drug Tariff, therefore Luforbec must be prescribed by brand to release savings for the NHS1
If you would like to know more about Luforbec and the savings opportunities available, please get in touch by email to:
UKRespiratory@lupin.com or call on: 01565 751378